Chemical species
French term | English term |
Substance chimique | Chemical substance |
Espèce chimique | Chemical species |
Espéce naturelle – espèce synthétique | Natural species – synthetic species |
Odeur – Odorat | Scent – Smell |
réactif | reagent |
Sulfate de cuivre anhydre | Anhydrous copper sulfate |
Gouttes | drops |
Dissoudre | Dissolve |
Tube à essai | Test tube |
Eau distillé | Distilled water |
Amidon | Starch |
Iode | iodine |
Acier galvanisé | Galvanized steel |
Le béton | Concrete |
Eau gazeuse | Carbonated water |
Eau de chaux | Lime water |
Un précipité | A precipitate |
La combustion | A combustion |
Le fer | Iron |
Le cuivre | Copper |
Graisses végétale hydrogénées | Hydrogenerated vegetable fat |
Solution acide | Acidic solution |
Solution basique | Basic solution |
Solution neutre | Neutral solution |
Une boisson | A beverage |
Les propriétés | Properties |
- Knowledge and skills
Differentiate between chemical substances and chemical species; natural chemical species and synthetic chemical species.
Propose tests when the five senses are not enough
I - Chemical species
1 - Definition
A chemical species is defined as a pure chemical substance characterized by constant physical properties. The chemical species can be a set of identical molecular, ionic, or atomic entities. Example:
Water consists of molecules with the formula H2
Iron consists of atoms with the formula Fe.
Salt or sodium chloride (NaCl) is composed of Na+ ions and Cl−
II - Natural and synthetic chemical species
- Natural chemical species are those that exist in nature.
- Synthetic chemical species are prepared by humans in laboratories or industrial facilities through chemical transformations.
- Artificial chemical species are synthetic chemical species that do not exist in nature.
III - Demonstration of certain chemical species existence
in Natural Products Most foods and everyday objects are composed of numerous chemical species. In chemistry, it is useful to be able to identify the chemical species present in a food or object.
Example: An apple contains water, sugar, colorants…
1 - Using our five senses
The first part of an analysis involves using your five senses: sight, touch, smell, hearing, and taste. However, our five senses are not always enough to identify all of the chemical species
Example: by cutting an apple, a student completed the following table:

- Notice
some products should neither be smelled nor tasted as they pose health risks.
2 - Trough certain chemical tests
When our senses do not allow us to identify a chemical species, specific tests must be conducted:
Simple Chemical Tests for the detection of certain chemical substances :
2-1 - Test for the presence of water using Anhydrous Copper Sulfate
Anhydrous copper sulfate is a white powder that turns blue in the presence of water.
Procedure:
- Using a spatula, place a small amount of anhydrous copper sulfate in a small dish.
- Add one to two drops of the substance to be tested.
Test Result: The test is positive if the white powder turns blue. The test is negative if the white powder does not turn blue.
2-2 - Test for the presence of Carbon Dioxide using Lime water
Lime water is a transparent liquid that becomes cloudy in the presence of carbon dioxide.
Procedure: Pass the gas to be tested through lime water.
Test Result: The test is positive if the lime water becomes cloudy. The test is negative if the lime water remains clear.
2-3 - Acidity test using Ph paper
When pH < 7, the solution is considered acidic. When pH = 7, the solution is considered neutral. When pH > 7, the medium is considered basic.
Note: When using a highly colored solution, the coloration of the solution can alter the color of the pH paper and therefore lead to an error in the deduced pH value.
2-3 - Glucose test using Fehling's solution
Fehling’s solution is a blue liquid that, when heated in the presence of certain sugars, produces a brick-red precipitate. This test is specific for the presence of certain sugars, such as glucose, which is a simple sugar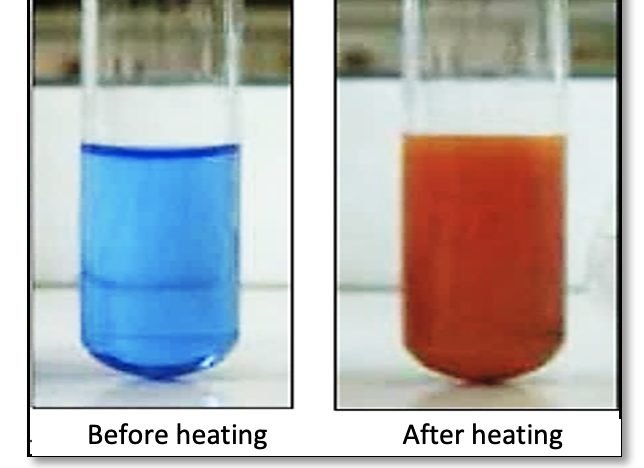
2-3 - Fat test using tracing paper
On tracing paper, fats leave visible greasy stains and marks.

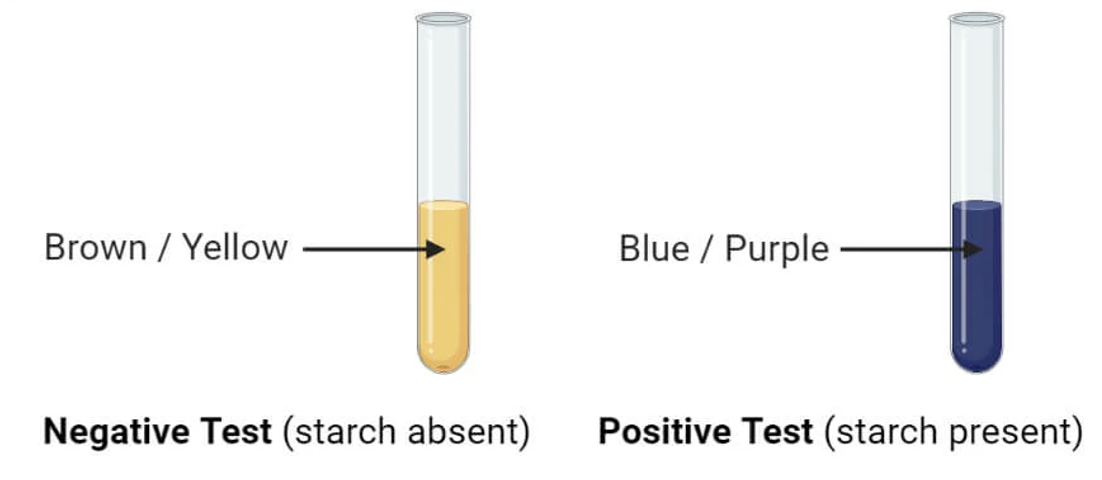
2-3 - Starch test using Iodine solution
Iodine solution is used to detect the presence of starch. In the presence of starch, the slightly brownish iodine solution turns a dark blue color. This dark blue color indicates the presence of starch, which is a complex carbohydrate found in foods like pasta and potatoes.
- Exercise
To highlight certain constituents of lemon juice, the following tests are conducted:
- A few drops of lemon juice are dripped onto anhydrous copper sulfate placed in a dish: the solid turns blue.
- Lemon zest is pinched near a candle flame: sparks appear in the flame.
- A few drops of lemon juice are dropped onto pH paper: a pH of 3.5 is found.
- A mixture of Fehling’s solution and lemon juice is warmed: a brick-red precipitate is obtained.
- What chemical species does test a. highlight?
- What can be said about the species highlighted by experiment b.?
- When tasting lemon juice, it is perceived as acidic but rarely sweet. Are these sensations in agreement with experiments c. and d.?
- Solution
- Test a highlights the presence of water in lemon juice.
- Experiment b suggests the presence of flammable organic compounds in lemon zest.
- The perception of lemon juice as acidic aligns with the result of experiment c (pH of 3.5). However, the perception of it as rarely sweet is not in agreement with experiment d, which indicates the presence of reducing sugars in lemon juice (brick-red precipitate with Fehling’s solution).